 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Dynamic Scan' found in 1 term [ ] and 2 definitions [ ] and 2 definitions [ ], (+ 16 Boolean[ ], (+ 16 Boolean[ ] results ] results
| previous 6 - 10 (of 19) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4] |  | |  | Searchterm 'Dynamic Scan' was also found in the following services: | | | | |
|  |  |
| |
|
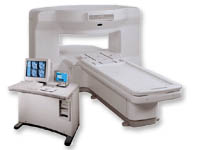
From GE Healthcare;
the New Signa Profile/i is a patient friendly open MRI system that virtually eliminates patient anxiety and claustrophobia, without compromising diagnostic utility.
Device Information and Specification CLINICAL APPLICATION Whole body Integrated transmit body coil, body flex sizes: M, L, XL, quadrature, head coil quadrature, 4 channel neurovascular array, 8 channel CTL array, quad. c- spine, 2 channel shoulder array, extremity coil, 3 channel wrist array, 4 channel breast array, 6, 9, 11 inch general purpose loop coils Standard: SE, IR, 2D/3D GRE and SPGR, Angiography: 2D/3D TOF, 2D/3D phase contrast; 2D/3D FSE, 2D/3D FRFSE, FGRE and FSPGR, SSFP, FLAIR, EPI, optional: 2D/3D Fiesta, fat/water separation, T1 FLAIRIMAGING MODES Localizer, single slice, multislice, volume, fast, POMP, multi slab, cine, slice and frequency zip, extended dynamic range, tailored RF TR 6 to 12000 msec in increments of 1 msec TE 1.3 to 2000 msec in increments of 1 msec 2D: 2.7mm - 20mm 3D: 0.2mm - 5mm 0.08 mm; 0.02 mm optional 10,000 kg w/gradient enclosure POWER REQUIREMENTS 200 - 480, 3-phase COOLING SYSTEM TYPE None required | |  | | | |
|  | |  |  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body SE, FE, IR, STIR, FFE, DEFFE, DESE, TSE, DETSE, Single shot SE, DRIVE, Balanced FFE, MRCP, Fluid Attenuated Inversion Recovery, Turbo FLAIR, IR-TSE, T1-STIR TSE, T2-STIR TSE, Diffusion Imaging, 3D SE, 3D FFE, Contrast Perfusion Analysis, MTC;; Angiography: CE-ANGIO, MRA 2D, 3D TOFOpen x 47 cm x infinite (side-first patient entry) POWER REQUIREMENTS 400/480 V | |  | |
• View the DATABASE results for 'Panorama 0.6T™' (2).
| | | | |
|  | |  |  |  |
| |
|
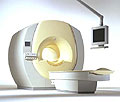
From Philips Medical Systems;
the Intera-family offers with this member a wide range of possibilities, efficiency and a ergonomic and intuitive serving-platform. Also available as Intera CV for cardiac and Intera I/T for interventional MR procedures.
The scanners are also equipped with SENSE technology, which is essential for high-quality contrast enhanced magnetic resonance angiography, interactive cardiac MR and diffusion tensor imaging ( DTI) fiber tracking.
The increased accuracy and clarity of MR scans obtained with this technology allow for faster and more accurate diagnosis of potential problems like patient friendliness and expands the breadth of applications including cardiology, oncology and interventional MR.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector; Optional SENSE coils: Flex-S-M-L, flex body, flex cardiac
SE, Modified-SE ( TSE), IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: PCA, MCA, Inflow MRA, CE
TR
2.9 (Omni), 1.6 (Power), 1.6 (Master/Expl) msec
TE
1.0 (Omni), 0.7 (Power), 0.5 (Master/Expl) msec
RapidView Recon. greater than 500 @ 256 Matrix
0.1 mm(Omni), 0.05 mm (Pwr/Mstr/Expl)
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for BOLD img.)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera 1.5T™' (2).
| | | | |
|  |  | Searchterm 'Dynamic Scan' was also found in the following services: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector, Optional SENSE Coils: Flex-S-M-L, Flex Body, Flex Cardiac
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
TR
Min. 2.9 (Omni) msec, 1.6 (Power) msec
TE
Min. 1.0 (Omni) msec, 0.7 (Power) msec
RapidView Recon. greater than 500 @ 256 Matrix
0.1 mm(Omni), 0.05 mm (Power)
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
STRENGTH
23 mT/m (Omni), 30 (Power) mT/m
| |  | |
• View the DATABASE results for 'Intera 1.0T™' (2).
| | | | |
|  | |  |  |  |
| |
|

It is important to remember when working around a superconducting magnet that the magnetic field is always on. Under usual working conditions the field is never turned off. Attention must be paid to keep all ferromagnetic items at an adequate distance from the magnet. Ferromagnetic objects which came accidentally under the influence of these strong magnets can injure or kill individuals in or nearby the magnet, or can seriously damage every hardware, the magnet itself, the cooling system, etc..
See MRI resources Accidents.
The doors leading to a magnet room should be closed at all times except when entering or exiting the room. Every person working in or entering the magnet room or adjacent rooms with a magnetic field has to be instructed about the dangers. This should include the patient, intensive-care staff, and maintenance-, service- and cleaning personnel, etc..
The 5 Gauss limit defines the 'safe' level of static magnetic field exposure. The value of the absorbed dose is fixed by the authorities to avoid heating of the patient's tissue and is defined by the specific absorption rate.
Leads or wires that are used in the magnet bore during imaging procedures, should not form large-radius wire loops. Leg-to-leg and leg-to-arm skin contact should be prevented in order to avoid the risk of burning due to the generation of high current loops if the legs or arms are allowed to touch. The patient's skin should not be in contact with the inner bore of the magnet.
The outflow from cryogens like liquid helium is improbable during normal operation and not a real danger for patients.
The safety of MRI contrast agents is tested in drug trials and they have a high compatibility with very few side effects. The variations of the side effects and possible contraindications are similar to X-ray contrast medium, but very rare. In general, an adverse reaction increases with the quantity of the MRI contrast medium and also with the osmolarity of the compound.
See also 5 Gauss Fringe Field, 5 Gauss Line, Cardiac Risks, Cardiac Stent, dB/dt, Legal Requirements, Low Field MRI, Magnetohydrodynamic Effect, MR Compatibility, MR Guided Interventions, Claustrophobia, MRI Risks and Shielding. | | | | | | | | |
• View the DATABASE results for 'MRI Safety' (42).
| | |
• View the NEWS results for 'MRI Safety' (13).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |