 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm '5 Gauss Fringe Field' found in 1 term [ ] and 7 definitions [ ] and 7 definitions [ ], (+ 14 Boolean[ ], (+ 14 Boolean[ ] results ] results
| previous 6 - 10 (of 22) nextResult Pages :  [1] [1]  [2] [2]  [3 4 5] [3 4 5] |  | | |  |  |  |
| |
|

It is important to remember when working around a superconducting magnet that the magnetic field is always on. Under usual working conditions the field is never turned off. Attention must be paid to keep all ferromagnetic items at an adequate distance from the magnet. Ferromagnetic objects which came accidentally under the influence of these strong magnets can injure or kill individuals in or nearby the magnet, or can seriously damage every hardware, the magnet itself, the cooling system, etc..
See MRI resources Accidents.
The doors leading to a magnet room should be closed at all times except when entering or exiting the room. Every person working in or entering the magnet room or adjacent rooms with a magnetic field has to be instructed about the dangers. This should include the patient, intensive-care staff, and maintenance-, service- and cleaning personnel, etc..
The 5 Gauss limit defines the 'safe' level of static magnetic field exposure. The value of the absorbed dose is fixed by the authorities to avoid heating of the patient's tissue and is defined by the specific absorption rate.
Leads or wires that are used in the magnet bore during imaging procedures, should not form large-radius wire loops. Leg-to-leg and leg-to-arm skin contact should be prevented in order to avoid the risk of burning due to the generation of high current loops if the legs or arms are allowed to touch. The patient's skin should not be in contact with the inner bore of the magnet.
The outflow from cryogens like liquid helium is improbable during normal operation and not a real danger for patients.
The safety of MRI contrast agents is tested in drug trials and they have a high compatibility with very few side effects. The variations of the side effects and possible contraindications are similar to X-ray contrast medium, but very rare. In general, an adverse reaction increases with the quantity of the MRI contrast medium and also with the osmolarity of the compound.
See also 5 Gauss Fringe Field, 5 Gauss Line, Cardiac Risks, Cardiac Stent, dB/dt, Legal Requirements, Low Field MRI, Magnetohydrodynamic Effect, MR Compatibility, MR Guided Interventions, Claustrophobia, MRI Risks and Shielding. | | | | | | | | | • For this and other aspects of MRI safety see our InfoSheet about MRI Safety. | | | • Patient-related information is collected in our MRI Patient Information.
| | | | | | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | MRI Safety Resources | | | | |
|  |  |  |
| |
|
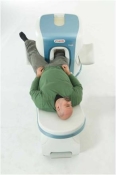
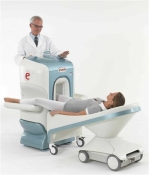
O-scan is manufactured and distributed by Esaote SpA
O-scan is a compact, dedicated extremity MRI system designed for easy installation and high throughput. The complete system fits in a 9' x 10' room, doesn't need for RF or magnetic shielding and it plugs in the wall. The 0.31T permanent magnet along with dual phased array RF coils, and advanced imaging protocols provide outstanding image quality and fast 25 minute complete examinations.
Esaote North America is the exclusive distributor of the O-scan system in the USA.
Device Information and Specification CLINICAL APPLICATION Dedicated Extremity
PULSE SEQUENCES
SE, HSE, HFE, GE, 2dGE, ME, IR, STIR, Stir T2, GESTIR, TSE, TME, FSE STIR, FSE ( T1, T2), X-Bone, Turbo 3DT1, 3D SHARC, 3D SST1, 3D SST2 2D: 2mm - 10 mm, 3D: 0.6 - 10 mm POWER REQUIREMENTS 100/110/200/220/230/240 | |  | | | |
|  | |  |  |  |
| |
|
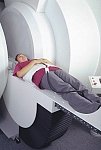
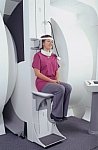
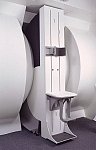
From FONAR Corporation;
in October of 2004, the company changed the product name of the Stand-Up™ MRI to the Upright™ MRI. The Indomitable™, Upright™ MRI is the only open MRI in the world that can perform positional MRI (pMRI), i.e. the Upright MRI™ scans patients in upright, weight-bearing positions, in addition to the conventional lie-down positions. The Upright™ MRI is the only device that can scan patients in the position their symptoms occur, in their position of pain. In early clinical reports independently confirm the effectiveness and potential of positional MRI. In October 2000, Fonar received permission to market the Indomitable™ from the FDA.
Device Information and Specification CLINICAL APPLICATION Whole body -
weight-bearing MRI -
position imaging (flexion, extension, bending, standing, sitting and recumbent scanning)
CONFIGURATION Front-open and Top-open MRIPOWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Water, closed-loop | |  | |
• View the DATABASE results for 'Upright™ MRI' (5).
| | |
• View the NEWS results for 'Upright™ MRI' (9).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
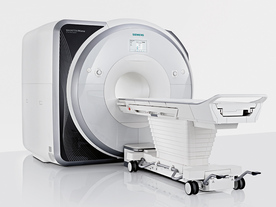
From Siemens Medical Systems;
Received FDA clearance in 2013.
The MAGNETOM Prisma is the 3T PowerPack for exploration that offers most demanding clinical and research challenges of today and the future. The latest parallel transmit technology, TimTX TrueShape, enables zooming into specific body regions for enhanced image quality. Furthermore, the Tim 4G integrated coil technology offers remarkable imaging flexibility and supports complex
examinations across the whole body.
Onsite upgrades to the MAGNETOM Prisma for customers who have already installed the 3 Tesla MAGNETOM Trio are possible.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
64, 128
MAGNET WEIGHT (gantry included)
13000 kg
DIMENSION H*W*D (gantry included)
173 x 230 x 222 cm
Passive, active; first order,
second order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground;
| |  | | | |
|  | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Cylindrical Wide Short Bore
Opt. (WIP) Single and Multi Voxel
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
TE
8 msec min. SE; 1.2 msec min. FE
less than 0.015 (256x256)
1.0 min. 2-DFT: 0.2 min. 3-DFT
32-1024, phase;; 64-1024, freq.
65.5 cm, patient aperture
40 50 kg (bare magnet incl. L-He)
COOLING SYSTEM TYPE
Closed-loop water-cooled
Liquid helium: approx. less than 0.05 L/hr
Passive, active, auto-active
| |  | |
• View the DATABASE results for 'Excelart AG™ with Pianissimo' (2).
| | | | |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |