 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'SPIR' was also found in the following services: | | | | |
|  |  |
| |
|
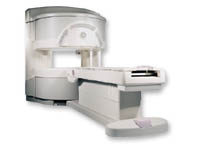
From GE Healthcare;
a friendly and less confining appearance targets the 7% of individuals who refuse to have an MRI because of claustrophobia. This open MRI system is also up to three times faster than other scanners, therefore the Signa OpenSpeed™ reducing exam time and scheduling
issues. In addition, a swing table provides better access and supports up to 500 pounds.
Device Information and Specification CLINICAL APPLICATION Whole body Standard: SE, IR, 2D/3D GRE and SPGR, Angiography: 2D/3D TOF, 2D/3D Phase Contrast;; 2D/3D FSE, 2D/3D FGRE and FSPGR, SSFP, FLAIR, EPI, optional: 2D/3D Fiesta, FGRET, Spiral, TensorTR 1.3 to 12000 msec in increments of 1 msec TE 0.4 to 2000 msec in increments of 1 msec 2D: 0.8mm - 20mm 3D: 0.1mm - 20mm 0.08 mm; 0.02 mm optional POWER REQUIREMENTS 200 - 480, 3-phase | |  | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
| |  | |
• View the DATABASE results for 'View' (160).
| | |
• View the NEWS results for 'View' (20).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
ABLAVAR™ (formerly named Vasovist™) is a blood pool agent for magnetic resonance angiography ( MRA), which opens new medical imaging possibilities in the evaluation of aortoiliac occlusive disease (AIOD) in patients with suspected peripheral vascular disease.
ABLAVAR™ binds reversibly to blood albumin, providing imaging with high spatial resolution up to 1 hour after injection, due to its high relaxivity and to the long lasting increased signal intensity of blood.
As with other contrast media: the possibility of serious or life-threatening anaphylactic or anaphylactoid reactions, including cardiovascular, re spiratory and/or cutaneous manifestations, should always be considered.
WARNING:
NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents increase the risk for nephrogenic systemic fibrosis (NSF) in patients with acute or chronic severe renal insufficiency (glomerular filtration rate less than 30 mL/min/1.73m 2), or acute renal insufficiency of any severity due to the hepato-renal syndrome or in the perioperative liver transplantation period.
See also Cardiovascular Imaging, Adverse Reaction, Molecular Imaging, and MRI Safety.
Drug Information and Specification
NAME OF COMPOUND
Diphenylcyclohexyl phosphodiester-Gd-DTPA, gadofosveset trisodium, MS-325
T1, predominantly positive enhancement
20-45 mmol-1sec-1, Bo=0,47T
PHARMACOKINETIC
Intravascular
CONCENTRATION
244 mg/mL, 0.25mmol/mL
DOSAGE
0.12 mL/kg, 0.03 mmol/kg
DEVELOPMENT STAGE
FDA approved
DO NOT RELY ON THE INFORMATION PROVIDED HERE, THEY ARE
NOT A SUBSTITUTE FOR THE ACCOMPANYING
PACKAGE INSERT!
Distribution Information
TERRITORY
TRADE NAME
DEVELOPMENT
STAGE
DISTRIBUTOR
USA, Canada, Australia
ABLAVAR™
Approved
| |  | |
• View the DATABASE results for 'ABLAVAR™' (3).
| | |
• View the NEWS results for 'ABLAVAR™' (1).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'SPIR' was also found in the following services: | | | | |
|  |  |
| |
|

From Hitachi Medical Systems America Inc.;
the AIRIS II, an entry in the diagnostic category of open MR systems, was designed by Hitachi
Medical Systems America Inc. (Twinsburg, OH, USA) and Hitachi Medical Corp. (Tokyo) and is manufactured by the Tokyo branch. A 0.3 T field-strength magnet and phased array coils deliver high image quality without the need for a tunnel-type high-field system, thereby significantly improving patient comfort not only for claustrophobic patients.
Device Information and Specification
CLINICAL APPLICATION
Whole body
QD Head, MA Head and Neck, QD C-Spine, MA or QD Shoulder, MA CTL Spine, QD Knee, Neck, QD TMJ, QD Breast, QD Flex Body (4 sizes), Small and Large Extrem., QD Wrist, MA Foot and Ankle (WIP), PVA (WIP)
SE, GE, GR, IR, FIR, STIR, FSE, ss-FSE, FLAIR, EPI -DWI, SE-EPI, ms - EPI, SSP, MTC, SARGE, RSSG, TRSG, MRCP, Angiography: CE, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 20 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 10 - 250msec IR: 10 -250msec GE: 5 - 50 msec FSE: 15 - 2,000
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
COOLING SYSTEM TYPE
Air-cooled
2.0 m lateral, 2.5 m vert./long
| |  | |
• View the DATABASE results for 'AIRIS II™' (2).
| | | | |
|  | |  |  |  |
| |
|
General MRI of the abdomen can consist of T1 or T2 weighted spin echo, fast spin echo ( FSE, TSE) or gradient echo sequences with fat suppression and contrast enhanced MRI techniques. The examined organs include liver, pancreas, spleen, kidneys, adrenals as well as parts of the stomach and intestine (see also gastrointestinal imaging). Respiratory compensation and breath hold imaging is mandatory for a good image quality.
T1 weighted sequences are more sensitive for lesion detection than T2 weighted sequences at 0.5 T, while higher field strengths (greater than 1.0 T), T2 weighted and spoiled gradient echo sequences are used for focal lesion detection.
Gradient echo in phase T1 breath hold can be performed as a dynamic series with the ability to visualize the blood distribution. Phases of contrast enhancement include the capillary or arterial dominant phase for demonstrating hypervascular lesions, in liver imaging the portal venous phase demonstrates the maximum difference between the liver and hypovascular lesions, while the equilibrium phase demonstrates interstitial disbursement for edematous and malignant tissues.
Out of phase gradient echo imaging for the abdomen is a lipid-type tissue sensitive sequence and is useful for the visualization of focal hepatic lesions, fatty liver (see also Dixon), hemochromatosis, adrenal lesions and renal masses.
The standards for abdominal MRI vary according to clinical sites based on sequence availability and MRI equipment.
Specific abdominal imaging coils and liver-specific contrast agents targeted to the healthy liver tissue improve the detection and localization of lesions.
See also Hepatobiliary Contrast Agents, Reticuloendothelial Contrast Agents, and Oral Contrast Agents.
For Ultrasound Imaging (USI) see Abdominal Ultrasound at Medical-Ultrasound-Imaging.com. | | | |  | |
• View the DATABASE results for 'Abdominal Imaging' (11).
| | |
• View the NEWS results for 'Abdominal Imaging' (3).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
|  |
Assessment of Female Pelvic Pathologies: A Cross-Sectional Study Among Patients Undergoing Magnetic Resonance Imaging for Pelvic Assessment at the Maternity and Children Hospital, Qassim Region, Saudi Arabia
Saturday, 7 October 2023 by www.cureus.com |  |  |
Higher Visceral, Subcutaneous Fat Levels Predict Brain Volume Loss in Midlife
Wednesday, 4 October 2023 by www.neurologyadvisor.com |  |  |
Deep Learning Helps Provide Accurate Kidney Volume Measurements
Tuesday, 27 September 2022 by www.rsna.org |  |  |
CT, MRI for pediatric pancreatitis interobserver agreement with INSPPIRE
Friday, 11 March 2022 by www.eurekalert.org |  |  |
Clinical trial: Using MRI for prostate cancer diagnosis equals or beats current standard
Thursday, 4 February 2021 by www.eurekalert.org |  |  |
Computer-aided detection and diagnosis for prostate cancer based on mono and multi-parametric MRI: A review - Abstract
Tuesday, 28 April 2015 by urotoday.com |  |  |
Nottingham scientists exploit MRI technology to assist in the treatment of IBS
Thursday, 9 January 2014 by www.news-medical.net |  |  |
New MR sequence helps radiologists more accurately evaluate abnormalities of the uterus and ovaries
Thursday, 23 April 2009 by www.eurekalert.org |  |  |
MRI identifies 'hidden' fat that puts adolescents at risk for disease
Tuesday, 27 February 2007 by www.eurekalert.org |
|
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |