 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'philips' found in 1 term [ ] and 14 definitions [ ] and 14 definitions [ ] ]
| previous 6 - 10 (of 15) nextResult Pages :  [1] [1]  [2 3] [2 3] |  | |  | Searchterm 'philips' was also found in the following services: | | | | |
|  |  |
| |
|
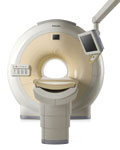
From Philips Medical Systems;
Philips continues to expand the frontiers of utra high field MRI with the introduction of the new Intera Achieva 3.0T™. Its powerful future-safe platform shares all the advantages of the Achieva family and covers applications throughout the whole body.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
Passive and dynamic, 1st order std./2nd opt.
| |  | | | | | | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body SE, FE, IR, STIR, FFE, DEFFE, DESE, TSE, DETSE, Single shot SE, DRIVE, Balanced FFE, MRCP, Fluid Attenuated Inversion Recovery, Turbo FLAIR, IR-TSE, T1-STIR TSE, T2-STIR TSE, Diffusion Imaging, 3D SE, 3D FFE, Contrast Perfusion Analysis, MTC;; Angiography: CE-ANGIO, MRA 2D, 3D TOFOpen x 47 cm x infinite (side-first patient entry) POWER REQUIREMENTS 400/480 V | |  | |
• View the DATABASE results for 'Panorama 0.6T™' (2).
| | | | |
|  | |  |  |  |
| |
|
 [This entry is marked for removal.]
[This entry is marked for removal.]
(July 20, 2009 - EPIX Pharmaceuticals, Inc. announced today that, in light of the company's lack of capital and inability to obtain additional financing or consummate a strategic transaction, it has entered into an Assignment for the Benefit of Creditors, effective immediately, in accordance with Massachusetts law).
EPIX has been a specialty pharmaceutical firm developing targeted contrast agents to improve the capability of MRI as a diagnostic tool for a variety of diseases. Gadofosveset trisodium (formerly MS-325, Vasovist™, now ABLAVARt™), is an injectable intravascular contrast agents designed for multiple vascular imaging applications, including peripheral vascular disease and coronary artery disease.
EPIX conducted a pivotal Phase III trial for the detection of peripheral vascular disease, as well as a Phase II feasibility trial for coronary artery disease diagnosis.
To ensure rapid development and adoption of gadofosveset trisodium into clinical practice upon regulatory approval, EPIX pursued an aggressive product development plan and commercialization strategy. The Company established an exclusive, worldwide sales and marketing agreement with Bayer Schering Pharma AG. EPIX also established corporate collaborations with GE Healthcare, Philips Medical Systems and Siemens Medical Systems, the three leading MRI manufacturers, which together account for approximately 80 percent of the MRI machines installed worldwide.
EPIX had other MRI contrast agents under development, most significantly a novel prototype blood clot agent ( EP-2104R). Potential clinical applications for this type of agent include detection of deep venous thrombosis, pulmonary embolism and blood clots in the coronary and carotid arteries. Currently, there is no high resolution imaging technique to directly visualize blood clots in patients with suspected cardiovascular disease.
| |  | |
• View the DATABASE results for 'EPIX Pharmaceuticals, Inc.' (7).
| | |
• View the NEWS results for 'EPIX Pharmaceuticals, Inc.' (69).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  |  | Searchterm 'philips' was also found in the following services: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector, Flex-S-M-L, flex body, flex cardiac, neuro-vascular, head
SE, Modified-SE ( TSE), DAVE, STIR, FLAIR, SPIR, MTC, Dynamic, Keyhole, CLEAR, Q Flow, Balanced FFE, Multi Chunk 3D, Multi Stack 3D, FFE-EPI, SE-EPI, IR-EPI, GRASE, Diffusion Imaging, Perfusion Imaging;; Angiography: Inflow MRA, TONE, PCA, CE MRA
RapidView Recon. greater than 500 @ 256 Matrix
128 x 128, 256 x 256,512 x 512,1024 x 1024
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera 0.5T™' (2).
| | | | |
|  | |  |  |  |
| |
|
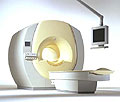
From Philips Medical Systems;
the Intera-family offers with this member a wide range of possibilities, efficiency and a ergonomic and intuitive serving-platform. Also available as Intera CV for cardiac and Intera I/T for interventional MR procedures.
The scanners are also equipped with SENSE technology, which is essential for high-quality contrast enhanced magnetic resonance angiography, interactive cardiac MR and diffusion tensor imaging ( DTI) fiber tracking.
The increased accuracy and clarity of MR scans obtained with this technology allow for faster and more accurate diagnosis of potential problems like patient friendliness and expands the breadth of applications including cardiology, oncology and interventional MR.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector; Optional SENSE coils: Flex-S-M-L, flex body, flex cardiac
SE, Modified-SE ( TSE), IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: PCA, MCA, Inflow MRA, CE
TR
2.9 (Omni), 1.6 (Power), 1.6 (Master/Expl) msec
TE
1.0 (Omni), 0.7 (Power), 0.5 (Master/Expl) msec
RapidView Recon. greater than 500 @ 256 Matrix
0.1 mm(Omni), 0.05 mm (Pwr/Mstr/Expl)
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for BOLD img.)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera 1.5T™' (2).
| | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |