 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Power' was also found in the following services: | | | | |
|  |  |
| |
|
The subacute risks and side effects of magnetic and RF fields (for patients and staff) have been intensively examined for a long time, but there have been no long-term studies following persons who have been exposed to the static magnetic fields used in MRI. However, no permanent hazardous effects of a static magnetic field exposure upon human beings have yet been demonstrated.
Temporary possible side effects of high magnetic and RF fields:
•
Varying magnetic fields can induce so-called magnetic phosphenes that occur when an individual is subject to rapid changes of 2-5 T/s, which can produce a flashing sensation in the eyes. This temporary side effect does not seem to damage the eyes. Static field strengths used for clinical MRI examinations vary between 0.2 and 3.0 tesla;; field changes during the MRI scan vary in the dimension of mT/s. Experimental imaging units can use higher field strengths of up to 14.0 T, which are not approved for human use.
•
The Radio frequency pulses mainly produce heat, which is absorbed by the body tissue. If the power of the RF radiation is very high, the patient may be heated too much. To avoid this heating, the limit of RF exposure in MRI is up to the maximum specific absorption rate (SAR) of 4 W/kg whole body weight (can be different from country to country). For MRI safety reasons, the MRI machine starts no sequence, if the SAR limit is exceeded.
•
Very high static magnetic fields are needed to reduce the conductivity of nerves perceptibly. Augmentation of T waves is observed at fields used in standard imaging but this side effect in MRI is completely reversible upon removal from the magnet. Cardiac arrhythmia threshold is typically set to 7-10 tesla. The magnetohydrodynamic effect, which results from a voltage occurring across a vessel in a magnetic field and percolated by a saline solution such as blood, is irrelevant at the field strengths used.
The results of some animal and cellular studies suggest the possibility that electromagnetic fields may act as co-carcinogens or tumor promoters, but the data are inconclusive.
Up to 45 tesla, no important effects on enzyme systems have been observed. Neither changes in enzyme kinetics, nor orientation changes in macromolecules have been conclusively demonstrated.
There are some publications associating an increase in the incidence of leukemia with the location of buildings close to high-current power lines with extremely low-frequency (ELF) electromagnetic radiation of 50-60 Hz, and industrial exposure to electric and magnetic fields but a transposition of such effects to MRI or MRS seems unlikely.
Under consideration of the MRI safety guidelines, real dangers or risks of an exposure with common MRI field strengths up to 3 tesla as well as the RF exposure during the MRI scan, are not to be expected.
For more MRI safety information see also Nerve Conductivity,
Contraindications, Pregnancy
and Specific Absorption Rate.
See also the related poll result: ' In 2010 your scanner will probably work with a field strength of' | |  | | • For this and other aspects of MRI safety see our InfoSheet about MRI Safety. | | | • Patient-related information is collected in our MRI Patient Information.
| | | | | | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | MRI Safety Resources | | | | |
|  |  |  |
| |
|

From ONI Medical Systems, Inc.;
MSK-Extreme™ MRI system is a dedicated high field extremity imaging device, designed to provide orthopedic surgeons and other physicians with detailed diagnostic images of the foot, ankle, knee, hand, wrist and elbow, all with the clinical confidence and advantages derived from high field, whole body MRI units. The light weight (less than 650 kg) of the OrthOne System performs rapid patient studies, is easy to operate, has a patient friendly open environment and can be installed in a practice office or hospital, all at a cost similar to a low field extremity machine.
New features include a more powerful operating system that offers increased scan speed as well as a 160-mm knee coil with higher signal to noise ratio, and the option of a CD burner.
Device Information and Specification 16 cm knee, 18 cm lower extremity;; 12.3 cm upper extremity, additional high resolution v-SPEC Coils: 80 mm, 100 mm, or 145 mm. SE, FSE, GE2D, GE3D, Inversion recovery (IR), Driven Equilibrium, Fat Saturation (FS), STIR, MT, PD, Flow Compensation (FC), RF spoiling, MTE, No Phase Wrap (NPW) IMAGING MODES Scout, single, multislice, volume 2D less than 200 msec/image X/Y: 64-512; 2 pixel steps 4,096 grey lvls; 256 lvls in 3D POWER REQUIREMENTS 115VAC, 1phase, 20A; 208VAC, 3 phase, 30A COOLING SYSTEM TYPE LHe with 2 stage cold head 1.25m radial x 1.8m axial | |  | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
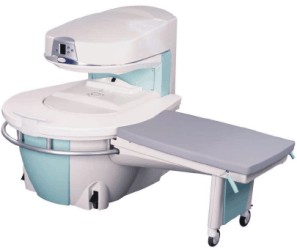
Manufactured by Esaote S.p.A.;
a low field open MRI scanner with permanent magnet for orthopedic use. The outstanding feature of this MRI system is a patient friendly design with 24 cm diameter, which allows the imaging of extremities and small body parts like shoulder MRI. The power consumption is around 1.3 kW and the needed minimum floor space is an area of 16 sq m.
At RSNA 2006 Hologic Inc. introduced a new dedicated extremity MRI scanner, the Opera. Manufactured by Esaote is the Opera a redesign of Esaote's 0.2 Tesla E-Scan XQ platform, which now enables complete imaging of all extremities, including hip and shoulder applications. 'Real-time positioning' reportedly speeds patient setup and reduces exam times.
Esaote North America and Hologic Inc are the U.S. distributors of this MRI device.
Device Information and Specification CLINICAL APPLICATION Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE IMAGING MODES Single, multislice, volume study, fast scan, multi slab2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm 4096 gray lvls, 256 lvls in 3D POWER REQUIREMENTS 2,0 kW; 110/220 V single phase | |  | |
• View the DATABASE results for 'Opera (E-SCAN™ XQ)' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Power' was also found in the following services: | | | | |
|  |  |
| |
|
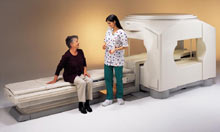
From Toshiba America Medical Systems Inc.;
the Ultra™ system was developed to help healthcare providers be more competitive by delivering greater patient comfort and a broad range of clinical capabilities, says Anita Bowler, product manager, MRI Business Unit, Toshiba America Medical Systems Inc With its unique, powerful gradient technology, the Ultra™ performs advanced clinical studies and consistently provides high-resolution images that are typically associated with high field MRI systems. At the same time, the Ultra™ offers a truly open feeling that makes patients more relaxed, especially those with claustrophobic tendencies.
Device Information and Specification CLINICAL APPLICATION Whole Body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI, Single shot EPI diffusion, True SSFP, SuperFASE; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC, Black Blood MRA POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | | | |
|  | |  |  |  |
| |
|

From Hitachi Medical Systems America Inc.;
the AIRIS II, an entry in the diagnostic category of open MR systems, was designed by Hitachi
Medical Systems America Inc. (Twinsburg, OH, USA) and Hitachi Medical Corp. (Tokyo) and is manufactured by the Tokyo branch. A 0.3 T field-strength magnet and phased array coils deliver high image quality without the need for a tunnel-type high-field system, thereby significantly improving patient comfort not only for claustrophobic patients.
Device Information and Specification
CLINICAL APPLICATION
Whole body
QD Head, MA Head and Neck, QD C-Spine, MA or QD Shoulder, MA CTL Spine, QD Knee, Neck, QD TMJ, QD Breast, QD Flex Body (4 sizes), Small and Large Extrem., QD Wrist, MA Foot and Ankle (WIP), PVA (WIP)
SE, GE, GR, IR, FIR, STIR, FSE, ss-FSE, FLAIR, EPI -DWI, SE-EPI, ms - EPI, SSP, MTC, SARGE, RSSG, TRSG, MRCP, Angiography: CE, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 20 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 10 - 250msec IR: 10 -250msec GE: 5 - 50 msec FSE: 15 - 2,000
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
COOLING SYSTEM TYPE
Air-cooled
2.0 m lateral, 2.5 m vert./long
| |  | |
• View the DATABASE results for 'AIRIS II™' (2).
| | | | |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |