 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
 [This entry is marked for removal.]
[This entry is marked for removal.]
General Electric (GE) agreed to buy diagnostic systems maker Lunar Corp. for $150m. in March 2000. In 2004/05 it seems that the integration process into GE Healthcare has been completed. (GE Medical Systems and Amersham announced in April 2004 the completion of a share exchange acquisition of Amersham Health by GE. The result of this acquisition is the new GE Healthcare, based in the UK, totally owned by General Electric (GE).
The U.S.-based company developed bone densitometers and scanning machines that measure bone density as a way of diagnosing osteoporosis and other metabolic bone diseases. GE Lunar marketed these products worldwide.
GE Lunar announced a distribution agreement with MagneVu for domestic sales of the MagneVu 1000, a portable MRI device for orthopedic use, under the trade name Applause™.
GE Lunar was the exclusive U.S. distributor of MR- devices manufactured by Esaote S.p.A. These compact in-office MRI™ machines are designed to fit all practice sizes in orthopedic imaging and complete the range of diagnostic imaging systems. | |  | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
| | | | | | | | |
• View the DATABASE results for 'Hardware' (19).
| | |
• View the NEWS results for 'Hardware' (1).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
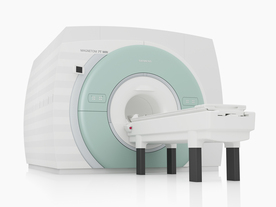
From Siemens Medical Systems;
The MAGNETOM 7T is designed as an open research platform. 7T MRI provides anatomical detail at the submillimiter scale, enhanced contrast mechanisms, outstanding spectroscopy performance, ultra-high resolution functional imaging ( fMRI), multinuclear whole-body MRI and functional information.
This ultra high field (UHF) MRI device is a research system and not cleared, approved or licensed in any jurisdiction for patient examinations.
Device Information and Specification
CLINICAL APPLICATION
Whole body
High-performance, ultra high field coils available. Integration and support for coil developments.
CHANNELS (min. / max. configuration)
32, optional 8 channels TX array
40 x 40 x 30 cm³ less than 8% nonlinearity
MAGNET WEIGHT (gantry included)
35017 kg
DIMENSION H*W*D (gantry included)
320 x 240 x 317,5 cm
MAX. AMPLITUDE
up to 70 mT/m
Up to 3rd order shim coils, user configurable B0 shim ? B0 maps and ROI definition
POWER REQUIREMENTS
2000 Volts, 650A
| |  | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|

It is important to remember when working around a superconducting magnet that the magnetic field is always on. Under usual working conditions the field is never turned off. Attention must be paid to keep all ferromagnetic items at an adequate distance from the magnet. Ferromagnetic objects which came accidentally under the influence of these strong magnets can injure or kill individuals in or nearby the magnet, or can seriously damage every hardware, the magnet itself, the cooling system, etc..
See MRI resources Accidents.
The doors leading to a magnet room should be closed at all times except when entering or exiting the room. Every person working in or entering the magnet room or adjacent rooms with a magnetic field has to be instructed about the dangers. This should include the patient, intensive-care staff, and maintenance-, service- and cleaning personnel, etc..
The 5 Gauss limit defines the 'safe' level of static magnetic field exposure. The value of the absorbed dose is fixed by the authorities to avoid heating of the patient's tissue and is defined by the specific absorption rate.
Leads or wires that are used in the magnet bore during imaging procedures, should not form large-radius wire loops. Leg-to-leg and leg-to-arm skin contact should be prevented in order to avoid the risk of burning due to the generation of high current loops if the legs or arms are allowed to touch. The patient's skin should not be in contact with the inner bore of the magnet.
The outflow from cryogens like liquid helium is improbable during normal operation and not a real danger for patients.
The safety of MRI contrast agents is tested in drug trials and they have a high compatibility with very few side effects. The variations of the side effects and possible contraindications are similar to X-ray contrast medium, but very rare. In general, an adverse reaction increases with the quantity of the MRI contrast medium and also with the osmolarity of the compound.
See also 5 Gauss Fringe Field, 5 Gauss Line, Cardiac Risks, Cardiac Stent, dB/dt, Legal Requirements, Low Field MRI, Magnetohydrodynamic Effect, MR Compatibility, MR Guided Interventions, Claustrophobia, MRI Risks and Shielding. | | | | | | | | |
• View the DATABASE results for 'MRI Safety' (42).
| | |
• View the NEWS results for 'MRI Safety' (13).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|

From ONI Medical Systems, Inc.;
MSK-Extreme™ MRI system is a dedicated high field extremity imaging device, designed to provide orthopedic surgeons and other physicians with detailed diagnostic images of the foot, ankle, knee, hand, wrist and elbow, all with the clinical confidence and advantages derived from high field, whole body MRI units. The light weight (less than 650 kg) of the OrthOne System performs rapid patient studies, is easy to operate, has a patient friendly open environment and can be installed in a practice office or hospital, all at a cost similar to a low field extremity machine.
New features include a more powerful operating system that offers increased scan speed as well as a 160-mm knee coil with higher signal to noise ratio, and the option of a CD burner.
Device Information and Specification 16 cm knee, 18 cm lower extremity;; 12.3 cm upper extremity, additional high resolution v-SPEC Coils: 80 mm, 100 mm, or 145 mm. SE, FSE, GE2D, GE3D, Inversion recovery (IR), Driven Equilibrium, Fat Saturation (FS), STIR, MT, PD, Flow Compensation (FC), RF spoiling, MTE, No Phase Wrap (NPW) IMAGING MODES Scout, single, multislice, volume 2D less than 200 msec/image X/Y: 64-512; 2 pixel steps 4,096 grey lvls; 256 lvls in 3D POWER REQUIREMENTS 115VAC, 1phase, 20A; 208VAC, 3 phase, 30A COOLING SYSTEM TYPE LHe with 2 stage cold head 1.25m radial x 1.8m axial | |  | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |