 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Cryogen' found in 2 terms [ ] and 59 definitions [ ] and 59 definitions [ ] ]
| previous 16 - 20 (of 61) nextResult Pages :  [1] [1]  [2 3 4 5 6 7 8 9 10 11 12 13] [2 3 4 5 6 7 8 9 10 11 12 13] |  | |  | Searchterm 'Cryogen' was also found in the following services: | | | | |
|  |  |
| |
|
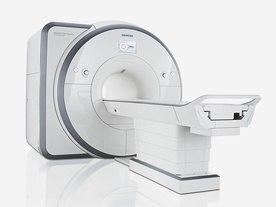
From Siemens Medical Systems;
Received FDA clearance in 2012.
The MAGNETOM Spectra is a cost-optimized high field MRI system with Tim 4G and Dot technologies. The system consumes less energy compared to other 3 Tesla scanners. The magnet-cooling helium is contained in a closed loop, which prevents the gas from escaping and reduces the need for refills. TimTX includes innovative techniques in the radio frequency excitation hardware as well as new application and processing features enabling uniform RF distribution in all body regions.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
Head, spine, torso/ body coil, neurovascular, neck and multi-purpose flex coils. Peripheral vascular, breast, shoulder, knee, wrist, foot//ankle, endorectal optional.
Chemical shift imaging, single voxel spectroscopy
DIMENSION H*W*D (gantry included)
173 x 231 x 219 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
Passive, active; first order standard, second order optional
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; connection value with chiller 100 kvA /without chiller 60 kVA
| |  | | | |
|  | |  |  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body SE, IR, 2D/3D TurboSE, Turbo IR, Dark-Fluid IR, True IR, 2D/3D MEDIC, 2D/3D GRE FLASH, 2D/3D GRE FISP, 2D/3D PSIF, 2D TurboFLASH, 3D MP-RAGE, 3D TurboFLASH, 2D/3D TOF angiography, MTC, TONE with 3D TOF MRA, GMR, LOTA IMAGING MODES Single, multislice, volume study, multi angle, multi oblique178 images/sec at 256 x 256 at 100% FOV1024 x 1024 full screen display POWER REQUIREMENTS 380/400/420/440/480 V Passive, act.; 1st order std./2nd opt. | |  | | | |
|  | |  |  |  |
| |
|

It is important to remember when working around a superconducting magnet that the magnetic field is always on. Under usual working conditions the field is never turned off. Attention must be paid to keep all ferromagnetic items at an adequate distance from the magnet. Ferromagnetic objects which came accidentally under the influence of these strong magnets can injure or kill individuals in or nearby the magnet, or can seriously damage every hardware, the magnet itself, the cooling system, etc..
See MRI resources Accidents.
The doors leading to a magnet room should be closed at all times except when entering or exiting the room. Every person working in or entering the magnet room or adjacent rooms with a magnetic field has to be instructed about the dangers. This should include the patient, intensive-care staff, and maintenance-, service- and cleaning personnel, etc..
The 5 Gauss limit defines the 'safe' level of static magnetic field exposure. The value of the absorbed dose is fixed by the authorities to avoid heating of the patient's tissue and is defined by the specific absorption rate.
Leads or wires that are used in the magnet bore during imaging procedures, should not form large-radius wire loops. Leg-to-leg and leg-to-arm skin contact should be prevented in order to avoid the risk of burning due to the generation of high current loops if the legs or arms are allowed to touch. The patient's skin should not be in contact with the inner bore of the magnet.
The outflow from cryogens like liquid helium is improbable during normal operation and not a real danger for patients.
The safety of MRI contrast agents is tested in drug trials and they have a high compatibility with very few side effects. The variations of the side effects and possible contraindications are similar to X-ray contrast medium, but very rare. In general, an adverse reaction increases with the quantity of the MRI contrast medium and also with the osmolarity of the compound.
See also 5 Gauss Fringe Field, 5 Gauss Line, Cardiac Risks, Cardiac Stent, dB/dt, Legal Requirements, Low Field MRI, Magnetohydrodynamic Effect, MR Compatibility, MR Guided Interventions, Claustrophobia, MRI Risks and Shielding. | | | | | | | | |
• View the DATABASE results for 'MRI Safety' (42).
| | |
• View the NEWS results for 'MRI Safety' (13).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Cryogen' was also found in the following services: | | | | |
|  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | |
• View the DATABASE results for 'OPART™' (2).
| | | | |
|  | |  |  | |  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |