 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Angiography' was also found in the following services: | | | | |
|  |  |
| |
|
Vasovistâ„¢ is an albumin-targeted intravascular contrast agent. It is indicated for contrast enhanced MR angiography (CE-MRA) for the visualization of abdominal or limb vessels in patients with suspected or known vascular disease. After IV injection, Vasovistâ„¢ binds reversibly to human albumin in plasma, which results in long-lasting increased relaxivity. Imaging from 5 to 50 min is possible. A small unbound portion is, by glomerular filtration, eliminated by the kidneys.
AngioMARK® was the formerly trade name and MS-325 the research name. Currently the phase III clinical trials are completed to determine its efficacy for peripheral vascular disease and coronary artery disease.
In the U.S., EPIX received an approvable letter from the U.S. Food and Drug Administration (FDA) for Vasovistâ„¢ in January 2005. In 2009, Epix Pharmaceuticals has sold the U.S., Canadian and Australian rights for its blood pool agent (now named ABLAVARâ„¢), to Lantheus Medical Imaging, Inc..
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents increase the risk for nephrogenic systemic fibrosis (NSF) in patients with acute or chronic severe renal insufficiency (glomerular filtration rate less than 30 mL/min/1.73m 2), or acute renal insufficiency of any severity due to the hepato-renal syndrome or the liver transplantation period.
See also MRI Safety.
Drug Information and Specification NAME OF COMPOUND Diphenylcyclohexyl phosphodiester-Gd-DTPA, gadofosveset trisodium, MS-325 T1, predominantly positive enhancement 20-45 mmol-1sec-1, Bo=0,47T PHARMACOKINETIC Intravascular, short elimination half life CONCENTRATION 244 mg/mL, 0.25mmol/mL DOSAGE 0.12 mL/kg, 0.03 mmol/kg DEVELOPMENT STAGE approved DO NOT RELY ON THE INFORMATION PROVIDED HERE, THEY ARE
NOT A SUBSTITUTE FOR THE ACCOMPANYING PACKAGE INSERT! Distribution Information TERRITORY TRADE NAME DEVELOPMENT
STAGE DISTRIBUTOR North America, Australia for sale | |  | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  |  | Searchterm 'Angiography' was also found in the following services: | | | | |
|  |  |
| |
|

From Hitachi Medical Systems America Inc.;
the AIRIS II, an entry in the diagnostic category of open MR systems, was designed by Hitachi
Medical Systems America Inc. (Twinsburg, OH, USA) and Hitachi Medical Corp. (Tokyo) and is manufactured by the Tokyo branch. A 0.3 T field-strength magnet and phased array coils deliver high image quality without the need for a tunnel-type high-field system, thereby significantly improving patient comfort not only for claustrophobic patients.
Device Information and Specification
CLINICAL APPLICATION
Whole body
QD Head, MA Head and Neck, QD C-Spine, MA or QD Shoulder, MA CTL Spine, QD Knee, Neck, QD TMJ, QD Breast, QD Flex Body (4 sizes), Small and Large Extrem., QD Wrist, MA Foot and Ankle (WIP), PVA (WIP)
SE, GE, GR, IR, FIR, STIR, FSE, ss-FSE, FLAIR, EPI -DWI, SE-EPI, ms - EPI, SSP, MTC, SARGE, RSSG, TRSG, MRCP, Angiography: CE, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 20 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 10 - 250msec IR: 10 -250msec GE: 5 - 50 msec FSE: 15 - 2,000
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
COOLING SYSTEM TYPE
Air-cooled
2.0 m lateral, 2.5 m vert./long
| |  | |
• View the DATABASE results for 'AIRIS II™' (2).
| | | | |
|  | |  |  |  |
| |
|
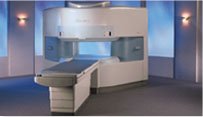
From Hitachi Medical Systems America, Inc.;
the AIRIS made its debut in 1995. Hitachi followed up with the AIRIS II system, which has proven equally successfully. 'All told, Hitachi has installed more than 1,000 MRI systems in the U.S., holding more than 17 percent of the total U.S. MRI installed base, and more than half of the installed base of open MR systems,' says Antonio Garcia, Frost and Sullivan industry research analyst.
Now Altaire employs a blend of innovative Hitachi features called VOSIâ„¢ technology, optimizing each sub-system's performance in concert with the
other sub-systems, to give the seamless mix of high-field performance
and the patient comfort, especially for claustrophobic patients, of open MR systems.
Device Information and Specification
CLINICAL APPLICATION
Whole body
DualQuad T/R Body Coil, MA Head, MA C-Spine, MA Shoulder, MA Wrist, MA CTL Spine, MA Knee, MA TMJ, MA Flex Body (3 sizes), Neck, small and large Extremity, PVA (WIP), Breast (WIP), Neurovascular (WIP), Cardiac (WIP) and MA Foot//Ankle (WIP)
SE, GE, GR, IR, FIR, STIR, ss-FSE, FSE, DE-FSE/FIR, FLAIR, ss/ms-EPI, ss/ms EPI- DWI, SSP, MTC, SE/GE-EPI, MRCP, SARGE, RSSG, TRSG, BASG, Angiography: CE, PC, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 3.6 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 8 - 250msec IR: 5.2 -7,680msec GE: 1.8 - 2,000 msec FSE: 5.2 - 7,680
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
COOLING SYSTEM TYPE
Water-cooled
3.1 m lateral, 3.6 m vertical
| |  | |
• View the DATABASE results for 'Altaire™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Angiography' was also found in the following services: | | | | |
|  |  |
| |
|
| |  | |
• View the DATABASE results for 'B22956' (2).
| | | | |
|  |  | Searchterm 'Angiography' was also found in the following services: | | | | |
|  |  |
| |
|
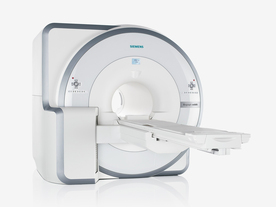
FDA cleared and CE Mark 2011.
The Biograph mMR has a fully-integrated design for simultaneous PET/MRI imaging. The dedicated hardware includes solid-state, avalanche photodiode PET detector and adapted, PET-compatible MR coils.
The possibility of truly simultaneous operation allows the acquisition of several magnetic resonance imaging ( MRI) sequences during the positron emission tomography (PET) scan, without increasing the examination time.
See also Hybrid Imaging.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Simultaneous PET/MRI
26 cm (typical overlap 23%)
A-P 45, R-L 50, H-F 50 cm
PET RING DIAMETER
65.6 cm
PATIENT SCAN RANGE
199 cm
HORIZONTAL SPEED
200 mmsec
PET DETECTOR
Solid state, 4032 avalanche photo diodes
DETECTOR SCINTILLATION MATERIAL
LSO, 28672 crystals
CRYSTAL SIZE
4 x 4 x 20 mm
DIMENSION H*W*D (gantry included)
335 x 230 x 242 cm (finshed covers)
COOLING SYSTEM
PET system: water; MRI system: water
Aautomatic, patient specific shim; active shim 3 linear and 5 non-linear channels (seond order)
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; Total system 110kW
| |  | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |