 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | '3%20phase%20liver%20mri' | |
Result : Searchterm '3 phase liver mri' found in 0 term [ ] and 0 definition [ ] and 0 definition [ ], (+ 15 Boolean[ ], (+ 15 Boolean[ ] results ] results
| 1 - 5 (of 15) nextResult Pages :  [1 2 3] [1 2 3] |  | | |  |  |  |
| |
|
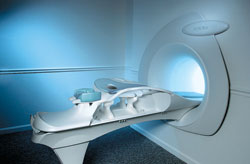
From Aurora Imaging Technology, Inc.;
The Aurora® 1.5T Dedicated Breast MRI System with Bilateral SpiralRODEO™ is the first and only FDA approved MRI device designed specifically for breast imaging. The Aurora System, which is already in clinical use at a growing number of leading breast care centers in the US, Europe, got in December 2006 also the approval from the State Food and Drug Administration of the People's Republic of China (SFDA).
'Some of the proprietary and distinguishing features of the Aurora System include: 1) an ellipsoid magnetic shim that provides coverage of both breasts, the chest wall and bilateral axillary lymph nodes; 2) a precision gradient coil with the high linearity required for high resolution spiral reconstruction;; 3) a patient-handling table that provides patient comfort and procedural utility; 4) a fully integrated Interventional System for MRI guided biopsy and localization; and 5) the user-friendly AuroraCAD™ computer-aided image display system designed to improve the accuracy and efficiency of diagnostic interpretations.'
Device Information and Specification
CONFIGURATION
Short bore compact
TE
From 5 ms for RODEO Plus to over 80 ms, 120 ms for T2 sequences
Around 0.02 sec for a 256x256 image, 12.4 sec for a 512 x 512 x 32 multislice set
20 - 36 cm, max. elliptical 36 x 44 cm
POWER REQUIREMENTS
150A/120V-208Y/ 3 Phase//60 Hz/5 Wire
| |  | | | | • Share the entry 'Aurora® 1.5T Dedicated Breast MRI System':    | | | | | | | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|

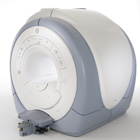
From Hitachi Medical Systems America Inc.;
the AIRIS II, an entry in the diagnostic category of open MR systems, was designed by Hitachi
Medical Systems America Inc. (Twinsburg, OH, USA) and Hitachi Medical Corp. (Tokyo) and is manufactured by the Tokyo branch. A 0. 3 T field-strength magnet and phased array coils de liver high image quality without the need for a tunnel-type high-field system, thereby significantly improving patient comfort not only for claustrophobic patients.
Device Information and Specification
CLINICAL APPLICATION
Whole body
QD Head, MA Head and Neck, QD C-Spine, MA or QD Shoulder, MA CTL Spine, QD Knee, Neck, QD TMJ, QD Breast, QD Flex Body (4 sizes), Small and Large Extrem., QD Wrist, MA Foot and Ankle (WIP), PVA (WIP)
SE, GE, GR, IR, FIR, STIR, FSE, ss-FSE, FLAIR, EPI -DWI, SE-EPI, ms - EPI, SSP, MTC, SARGE, RSSG, TRSG, MRCP, Angiography: CE, 2D/ 3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 20 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 10 - 250msec IR: 10 -250msec GE: 5 - 50 msec FSE: 15 - 2,000
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
COOLING SYSTEM TYPE
Air-cooled
2.0 m lateral, 2.5 m vert./long
| |  | |
• View the DATABASE results for 'AIRIS II™' (2).
| | | | |
|  | |  |  |  |
| |
|
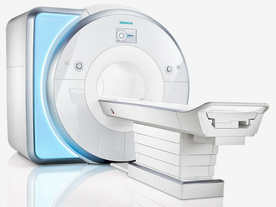
From GE Healthcare;
The Signa HDx MRI system is GE's leading edge whole body magnetic resonance scanner designed to support high resolution, high signal to noise ratio, and short scan times.
Signa HDx 3.0T offers new technologies like ultra-fast image reconstruction through the new XVRE recon engine, advancements in parallel imaging algorithms and the broadest range of premium applications. The HD applications, PROPELLER (high-quality brain imaging extremely resistant to motion artifacts), TRICKS (contrast-enhanced angiographic vascular lower leg imaging), VIBRANT (for breast MRI), LAVA (high resolution liver imaging with shorter breath holds and better organ coverage) and MR Echo (high-definition cardiac images in real time) offer unique capabilities.
Device Information and Specification CLINICAL APPLICATION Whole body
CONFIGURATION Compact short bore SE, IR, 2D/ 3D GRE, RF-spoiled GRE, 2DFGRE, 2DFSPGR, 3DFGRE, 3DFSPGR, 3DTOFGRE, 3DFSPGR, 2DFSE, 2DFSE-XL, 2DFSE-IR, T1-FLAIR, SSFSE, EPI, DW-EPI, BRAVO, Angiography: 2D/ 3D TOF, 2D/ 3D phase contrast vascular IMAGING MODES Single, multislice, volume study, fast scan, multi slab, cine, localizer H*W*D 240 x 2216,6 x 201,6 cm POWER REQUIREMENTS 480 or 380/415, 3 phase ||
COOLING SYSTEM TYPE Closed-loop water-cooled grad. | |  | | | |
|  | |  |  |  |
| |
|
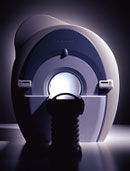
From Siemens Medical Systems;
Received FDA clearance in 2010.
MAGNETOM Skyra is a top-of-the-line, patient friendly wide bore 3 Tesla MRI system.
The system is equipped with the Tim 4G and Dot system (Total imaging matrix and Day optimizing throughput), to enhance both productivity and image quality with the complete range of advanced applications for clinical routine and research. Tim 4G features lighter, trimmer MRI coils that take up less space inside the magnet but de liver a high coil element density with increased signal to noise ratio and the possibility to use high iPAT factors.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
48, 64, 128
Chemical shift imaging, single voxel spectroscopy
MINIMUM TE
3D T1 spoiled GRE: 0.22 (256 matrix), Ultra-short TE
At isocenter: L-R 70 cm, A-P (with table) 55 cm
MAGNET WEIGHT (gantry included)
5768 kg
DIMENSION H*W*D (gantry included)
173 x 231 x 219 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
3 linear with 20 coils, 5 nonlinear 2nd-order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 110 kVA
| |  | | | |
|  | |  |  |  |
| |
|
From Toshiba America Medical Systems Inc.;
With its high-field strength, the Vantage™ de livers the clinical capabilities and image quality expected by cardiologists, while simultaneously offering patients a more comfortable and non-invasive option, said Dane Peshe, director, MRI Business Unit, Toshiba America Medical Systems. Vantage™ supports a full complement of cardiovascular imaging studies, ranging from stroke evaluation to peripheral vascular imaging. Additionally, the ultra short bore design offers patients a greater feeling of openness that reduces claustrophobic sensations, while Toshiba's exclusive, patented Pianissimo™ technology reduces scan noise by as much as 90 percent for a more pleasant experience.'
Device Information and Specification CLINICAL APPLICATION Whole body CONFIGURATION Ultra short bore SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, EPI, SuperFASE; Angiography: 2D(gate/non-gate)/ 3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study 32-1024, phase;; 64-1024, freq. POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Closed-loop water-cooled Liquid helium: approx. less than 0.05 L/hr Passive, active, auto-active | |  | |
• View the DATABASE results for 'Vantage™' (2).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |
|  | |
|  | 1 - 5 (of 15) nextResult Pages :  [1 2 3] [1 2 3] |
| |
|
| |
 | Look
Ups |
| |